| Strain Name |
NOD.CB17-PrkdcscidIl2rgtm1/Bcgen |
Common Name |
B-NDG mice |
| Background |
NOD-scid |
Catalog number | 110586 |
| Aliases | Male: Prkdc (-/-), Il2rg (X-/Y); Female: Prkdc (-/-), Il2rg (X-/X-) | ||
|
NCBI Gene ID |
16186 | ||
PDX models are successfully established in B-NDG mice
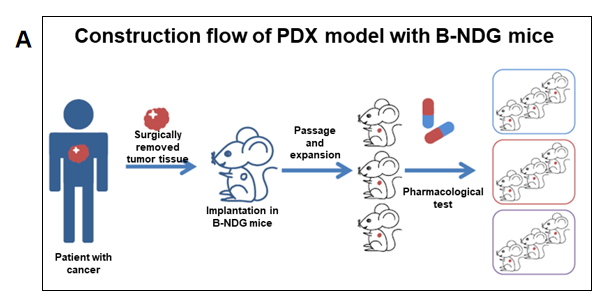
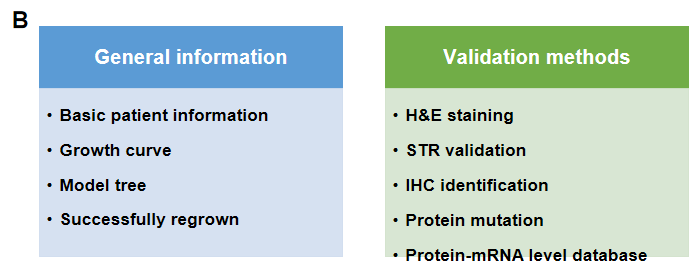
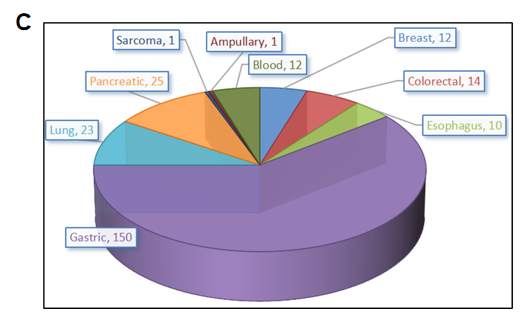
PDX (Patient-derived xenograft) models were successfully established in B-NDG mice. (A) General construction flow of the PDX model; (B) General information of tumor to be collected and the validation methods for the PDX model. (C) 248 PDX models derived from 9 types of tumors have been successfully established with B-NDG mice in Biocytogen by now.
Growth curve and STR identification for different generation of PDX tumor
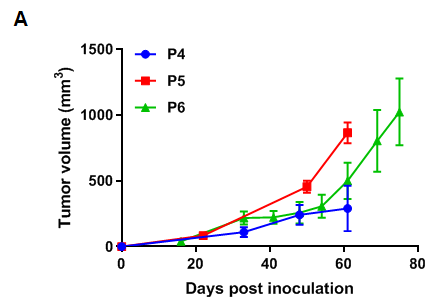
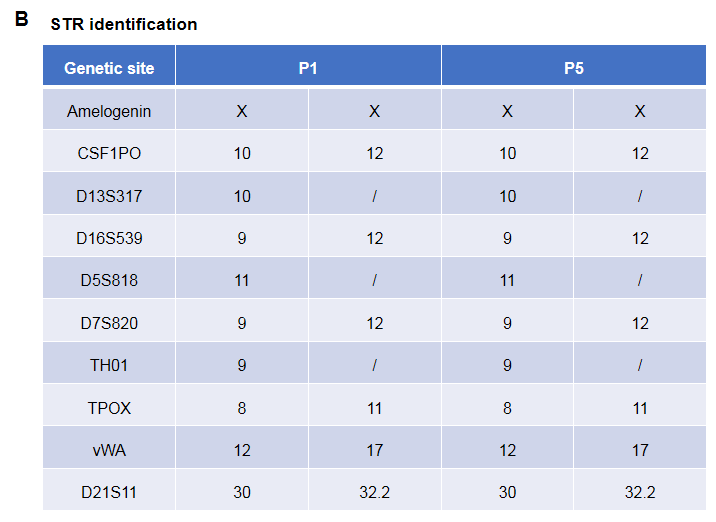
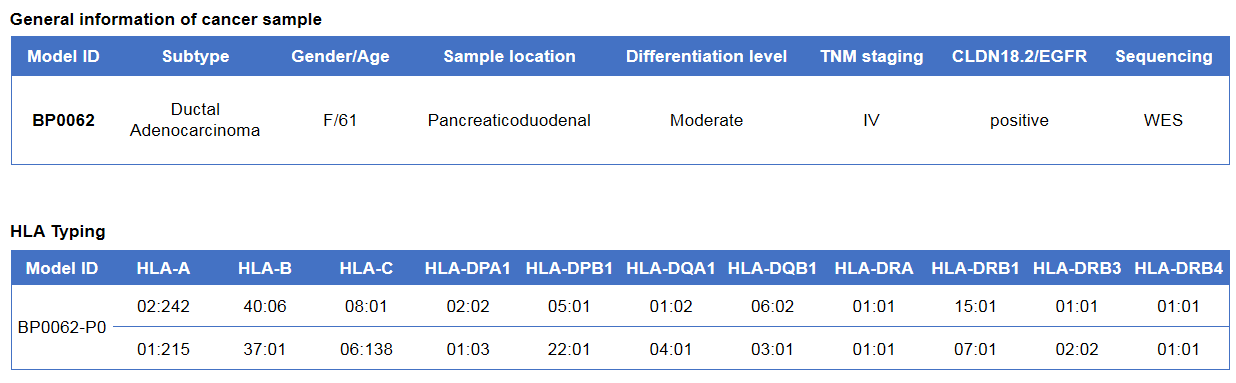
Pancreatic cancer BP0062 can successfully establish PDX model. Primary tumor sample was obtained from patient undergoing surgery for pancreatic ductal adenocarcinoma. Tumor pieces were subcutaneously inoculated into B-NDG mice. (A) Growth curve of passage 4, 5, 6 of the PDX tumor; (B) STR identification results of passage 1 and passage 5. Results showed that PDX model was successfully established in B-NDG mice implanted with pancreatic cancer BP0062. The STR of 5th generation of tumor cells is 100% matched to that of 1st generation, indicating that there was no change in the genetic background of the 5th generation of tumor tissue. Values are expressed as mean ± SEM.
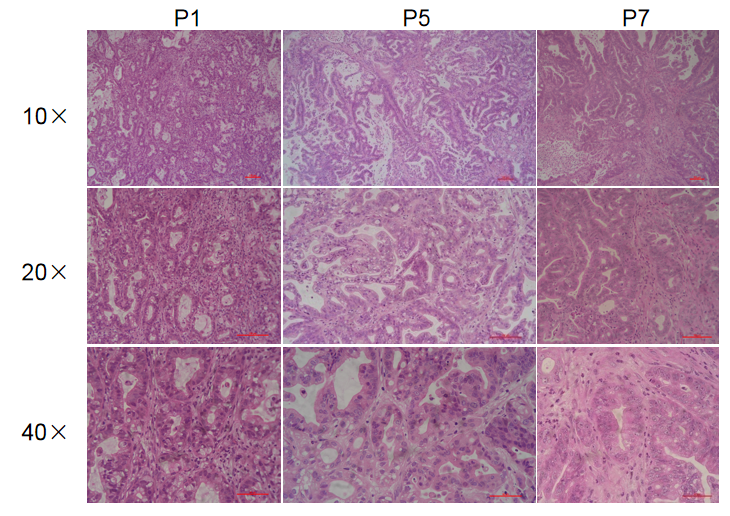
Histopathological analysis of BP0062 PDX tumor in B-NDG mice. Tumor tissue was collected from PDX model established with Pancreatic cancer BP0062 and analyzed with H&E staining. Results showed that patient-derived xenografts were found to well recapitulate the structures in original patient sample and maintain similar heterogeneity in different generations.
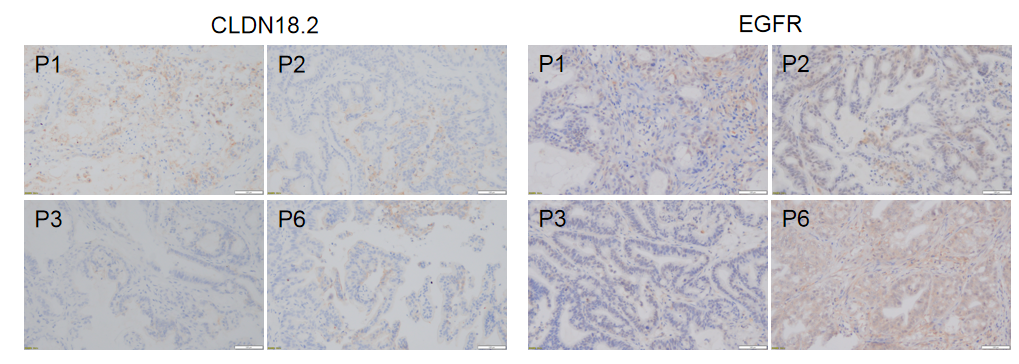
Immunohistochemical analysis of CLDN18.2 and EGFR in different generation of BP0062 PDX tumor tissue. Human CLDN18.2 and EGFR were respectively detected with anti-human CLDN18.2 antibody or anti-human EGFR antibody. The results showed that the number and distribution of CLDN18.2+ cells or EGFR+ cells in different generation of PDX tumor were obviously different (brown), indicating that the heterogeneity was maintained in different generation of PDX models.
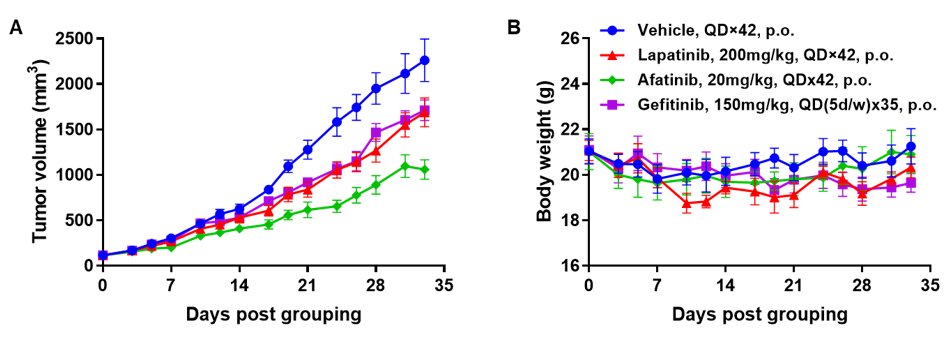
Antitumor activity of EGFR targeted drugs in PDX model BP0062 of pancreatic cancer established with B-NDG mice. (A) Molecular targeted small-molecule anti-cancer drugs slightly inhibited tumor growth of BP0062 in B-NDG mice. PDX tumor pieces of BP0062 were subcutaneously implanted into B-NDG mice (female, 6 week-old, n=6). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with different targeted drugs and schedules indicated in panel (B) Body weight changes during treatment. As shown in panel A, Molecular targeted small-molecule anti-cancer drugs were efficacious, demonstrating that PDX model of BP0062 can be used to establish tumor model and provide a powerful preclinical pancreatic tumor model with EGFR positive cells. Values are expressed as mean ± SEM.
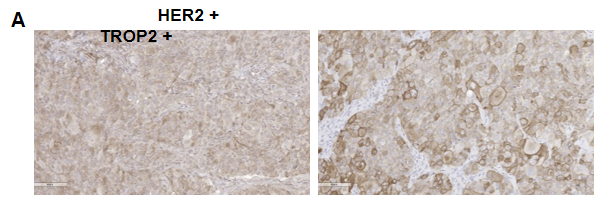
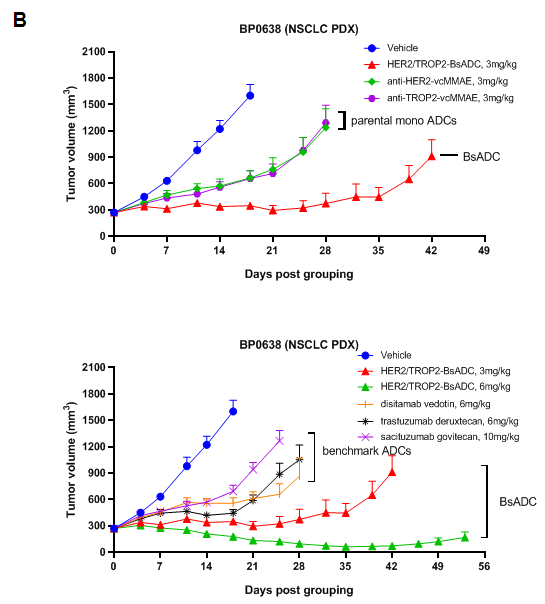
Antitumor activity of HER2-targeting and TROP2-targeting ADC drugs in NSCLC PDX model. (A) Expression analysis of HER2 and TROP2 in NSCLC (Non small Cell Lung Cancer) PDX (BP0638) tumor tissue by immunohistochemistry. The results showed both HER2 and TROP were low expression in this PDX model. (B) Efficacy verification of ADC drugs. PDX tumor pieces of BP0638 were subcutaneously implanted into B-NDG mice (n=5). Mice were grouped when tumor volume reached approximately 250-300 mm3, at which time they were treated with (HER2/TROP2-BsADC), two parental mono ADCs of the BsADC and other three commercial-derived ADC drugs (sacituzumab govitecan, disitamab vedotin, trastuzumab deruxtecan). The results showed that HER2/TROP2-BsADC had a stronger and longer inhibitory effect on tumor growth than ADCs. This inhibition was dose-dependent. Therefore, NSCLC PDX model of BP0062 provides a powerful preclinical NSCLC model for efficacy evaluation of various types of ADC drugs. Values are expressed as mean ± SEM.
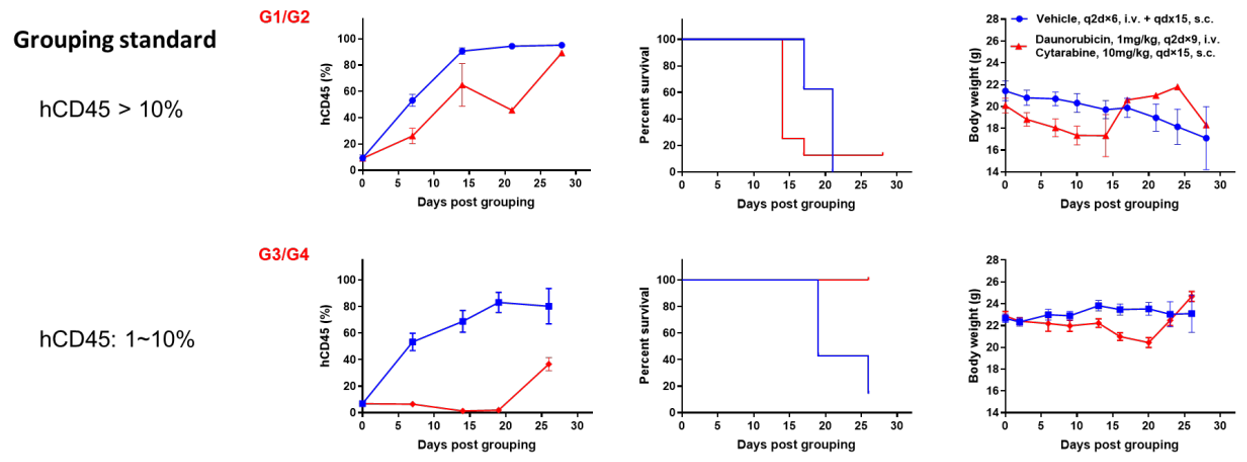
Antitumor activity of small molecule drugs in AML PDX model BP2010 established with B-NDG mice. According to the proportion of hCD45 in peripheral blood, PDX model mice were divided into 2 groups. One group of mice had a proportion of hCD45 in blood greater than 10% and the proportion of hCD45 in the blood of the other group of mice was between 1% and 10%. Each group was then divided into two sub-groups: the vehicle group and the treatment group. Mice in the treatment group were injected intravenously with daunorubicin and subcutaneously with cytarabine. The results showed that the efficacy of the group with low proportion of hCD45 (1%-10%) was better than that of the group with high proportion of hCD45 (>10%). Therefore, AML PDX model of BP2010 provides a powerful preclinical AML model for efficacy evaluation of chemotherapy drugs. Values are expressed as mean ± SEM.
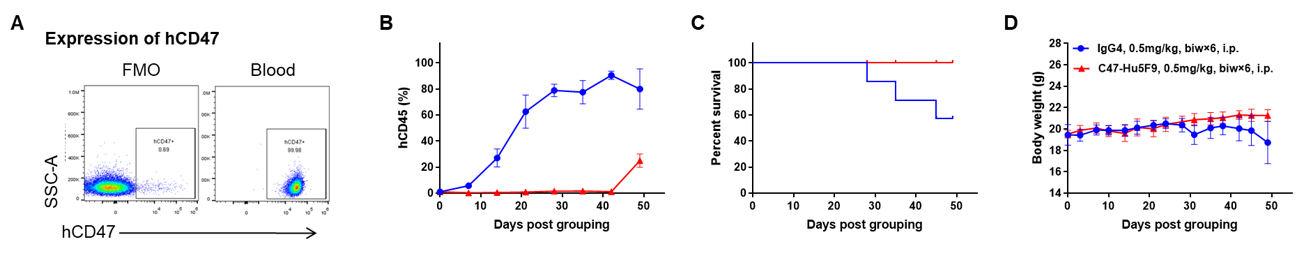
Antitumor activity of anti-human CD47 antibody in AML PDX model BP2010 established with B-NDG mice. (A) Human CD47 was highly expressed on the surface of tumor cells of BP2010; (B) The proportion of hCD45+ cells in the anti-human CD47 antibody treatment group was significantly reduced. PDX tumor cells of BP2010 were injected into B-NDG mice via tail vein (female, n=7). Mice were grouped when hCD45+ cells of PB reached approximately 1%, at which time they were treated with anti-human CD47 antibody Hu5F9 (in house) and schedules indicated in panel; (C) The survival rate of mice in the treatment group was significantly improved; (D) Body weight changes during treatment. The results indicated that AML PDX model of BP2010 provides a powerful preclinical AML model for efficacy evaluation of anti-human CD47 antibodies. Values are expressed as mean ± SEM.











